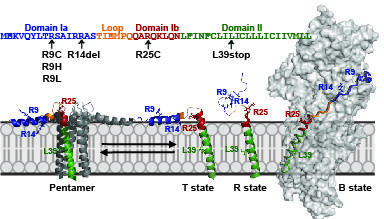
Calcium re-uptake to the sarcoplasmic reticulum (SR) for muscle relaxation is primarily mediated by the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA). SERCA’s ability to transport Ca2+ is modulated by two transmembrane proteins, phospholamban (PLN) and sarcolipin (SLN) depending on the tissue type. By employing magic-angle-spinning (MAS) and oriented solid-state NMR methods we aim to understand the unique regulatory mechanisms of PLN and SLN, and how their regulation of SERCA is perturbed by post-translational modifications and pathogenic mutations.